Welcome to KVS Technologies
KVS Technologies has a strong professional experience of CSV and regulatory
services in Healthcare, Pharmaceuticals and Life sciences industries to meet
customer expectations. KVS was founded by Mr. Shankar Sapavadiya and
Mr. KalpeshKumar Vaghela in Year 2006. Both promoters have 30+ Years of
experience in the fields of Instrumentation , Automation and Validation in Pharma
Industries. The main area of interest is to provide CSV and compliance services to Life
science, Healthcare and Pharmaceutical Industries of India and worldwide.
KVS Team has 16+ years of experience of Validation of computerised Systems, IT
Systems. We build the most effective environment for customers on current updates
of 21 CFR Part 11, EU Annex11 regulatory Compliance. KVS has provided CSV and
regulatory compliance services to more than 150 companies in India and worldwide
in the fields of life sciences, Pharmaceuticals, Chemicals, Medical Device, cosmetics
Why Choose KVS Technologies
As KVS Technologies we work as Your Validation Partner, we work with commitments. Our rooted knowledge with the industries, stems from our continuous learning, a key to our market leadership.
BMS Validation System
BMS Validation System, Sauter, Honeywell, Siemens, Johnsons.
Clinical Research Software
– Clinical Trial application software, PE Access.
– SAS, WIN Non Lin, Application Software Validation.
Regulatory compliances Services
Contract Laboratory or commercial testing labs or third party testing labs are driven by their customer and market demands chemical.
Preparation of SOPS
To lay down a procedure for the preparation, approval, authorization, control and revision of Standard Operating Procedures.
Validations
Establishing documented evidence that provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specificatios and quality attributes.
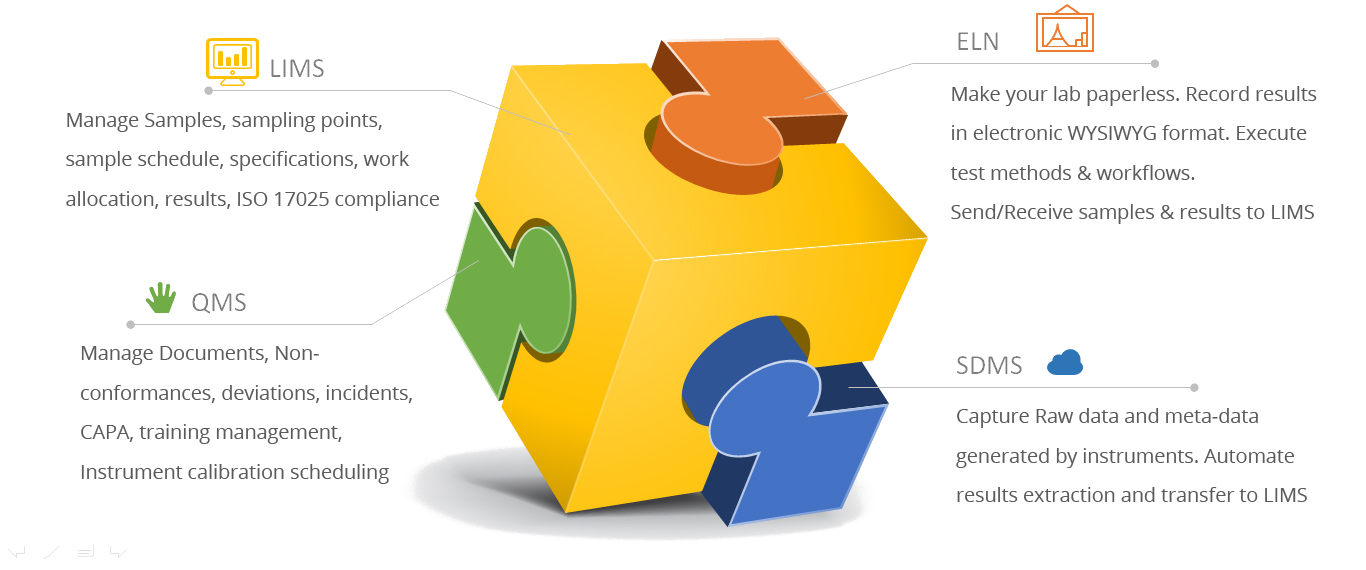
Lab Equipments Validation
– HPLC, GC, FTIR Stability Chamber Software – Enviro
– Laboratory Information Management System (LIMS) and Computer System.
– Application Software Validation
Some of Our Clients
We have supplied our products and services to clients as per their requirements. Our major clients are